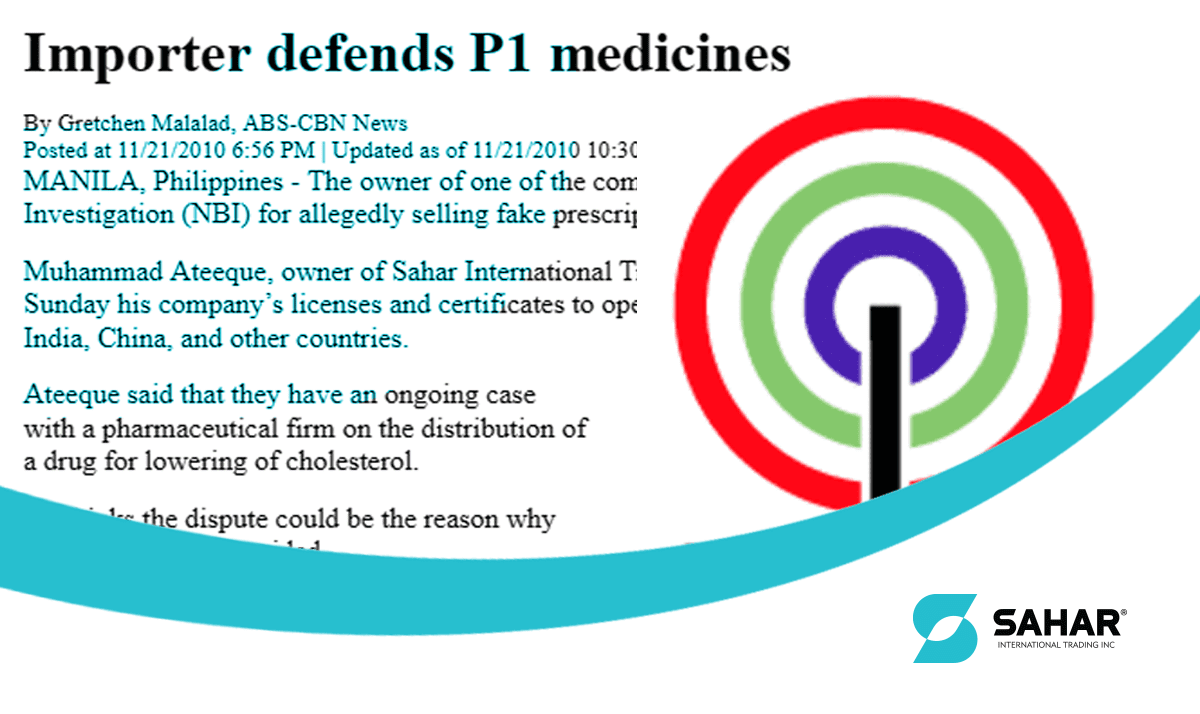
In a move to intensify the implementation of the cheaper medicine law, the Department of Justice (DOJ) dismissed the case of counterfeiting filed by three multi-national companies against a local drug distributor.
In the 6-page resolution written by Assistant State Prosecutor Cesar Calubag, no sufficient evidence was found to prosecute Renante Dumasig for drug forgery based on the case filed by Astra Zeneca, Pfiezer Philippines and Novartis. Assistant Chief Prosecutor Pedrito Lances, DOJ chairman of Task Force on Anti intellectual Property Piracy, recommended the approval of the resolution which was approved by the Prosecutor General Claro Arellano. The DOJ panel said “although the complainants proved that the drugs confiscated from Dumasig on November 18, 2010 were not registered with the Food and Drug Administration (FDA), they were not even proven to be fake.” In their investigation of the samples submitted by the three companies, the drugs were found to be manufactured and registered in other countries. “This proves why it is not registered with the Philippine FDA and its labels are not in accordance with the Philippine Generic Labeling Requirements. This is not evidence of fake medicine.”
In the 6-page resolution written by Assistant State Prosecutor Cesar Calubag, no sufficient evidence was found to prosecute Renante Dumasig for drug forgery based on the case filed by Astra Zeneca, Pfiezer Philippines and Novartis. Assistant Chief Prosecutor Pedrito Lances, DOJ chairman of Task Force on Anti intellectual Property Piracy, recommended the approval of the resolution which was approved by the Prosecutor General Claro Arellano. The DOJ panel said “although the complainants proved that the drugs confiscated from Dumasig on November 18, 2010 were not registered with the Food and Drug Administration (FDA), they were not even proven to be fake.” In their investigation of the samples submitted by the three companies, the drugs were found to be manufactured and registered in other countries. “This proves why it is not registered with the Philippine FDA and its labels are not in accordance with the Philippine Generic Labeling Requirements. This is not evidence of fake medicine.”