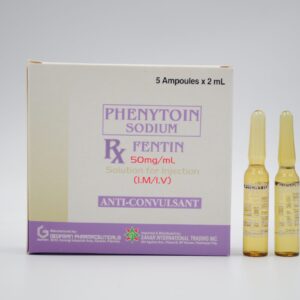
| GENERIC NAME: Pantoprazole (as Sodium Sesquihydrate) |
| BRAND NAME: Sitipraz |
| DOSAGE STRENGTH : 40 mg |
| DOSAGE FORM : Lyophilized Powder for Injection (IV) |
| PHARMACOLOGIC CATEGORY: Proton Pump Inhibitor |
| INDICATIONS: It is indicated for the treatment of gastro-oesophageal reflux disease, peptic ulcer disease, and Zollinger-ellison syndrome |
| PACKAGING: USP Type I clear, Colourless Glass Vial + 0.9 % Sodium Chloride Injection (10 mL) |
| REG Number: DRP-4060 |
|
|