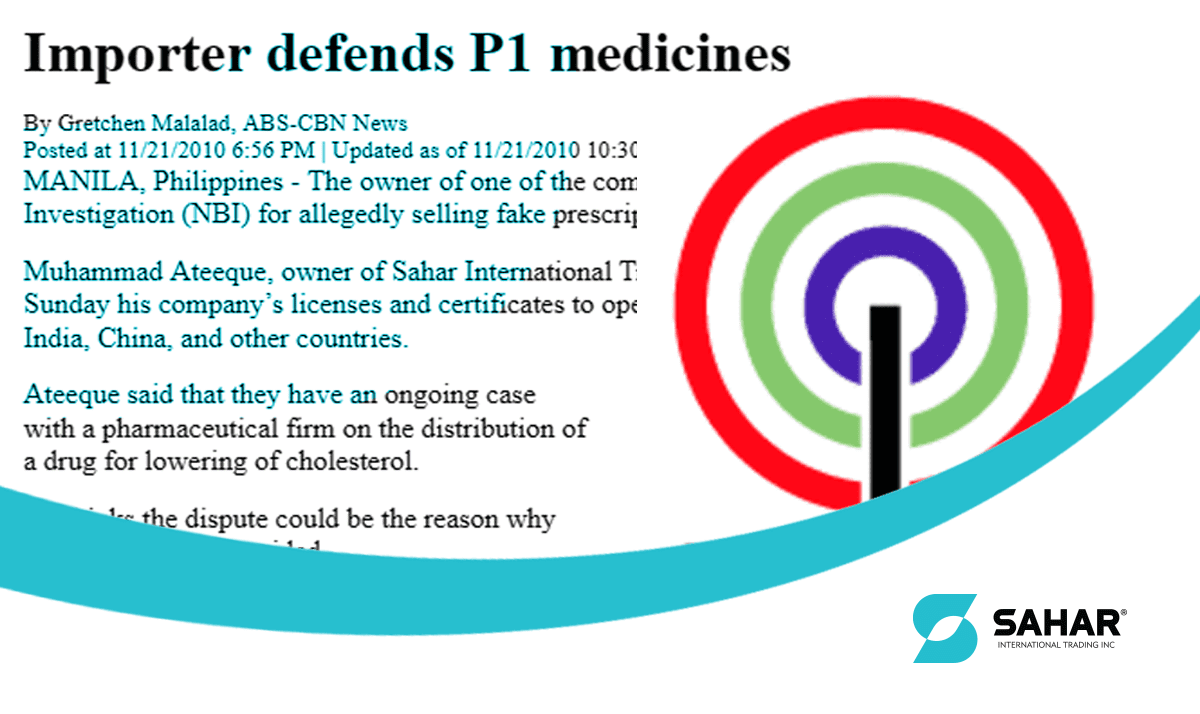
Several small drug companies have accused multinational firms of obstructing the implementation of Republic Act 9502, also known as the Universally Accessible Cheaper and Quality Medicine Act of 2008. The multinational firms have filed a case against these small companies, alleging a violation of patent protection laws. Recently, Femma Drug, Mark Erickson Enterprises, and Ellebasy Medical Trading were raided by the police and agents of the National Bureau of Investigation (NBI) in connection with a warrant and seizure order obtained by Merck & Dome. The order was for alleged infringement of patent rights and illegal sale of medicine. However, the three companies have denied these allegations and claimed that they were selling generic drugs.
This situation highlights the ongoing struggle between small drug companies and multinational firms in the pharmaceutical industry. The implementation of Republic Act 9502 was meant to provide affordable and accessible medicine to the public, but it seems that some companies are trying to prevent this from happening. The raid on these small companies is a clear indication of the lengths that some multinational firms will go to protect their profits.
It is important to note that the three companies in question have denied any wrongdoing and have claimed that they were selling generic drugs. This raises questions about the validity of the allegations made by Merck & Dome and the motives behind their actions. It is crucial that a fair and impartial investigation is conducted to determine the truth of the matter.
In conclusion, the pharmaceutical industry is a complex and highly competitive field, and the ongoing struggle between small drug companies and multinational firms is a clear example of this. However, the recent controversy surrounding Merck & Dome’s allegations has cast a shadow over the industry’s reputation.
The public’s trust in pharmaceutical companies has been shaken, and it is up to the industry leaders to restore it. This can only be achieved through transparency, accountability, and a commitment to putting the public’s health first. The implementation of Republic Act 9502 was meant to provide affordable and accessible medicine to the public, and any attempts to obstruct this should be thoroughly investigated. It is essential that all parties involved act in a professional and ethical manner to ensure that the public’s health and well-being are not compromised.
The public’s trust in pharmaceutical companies has been shaken, and it is up to the industry leaders to restore it. This can only be achieved through transparency, accountability, and a commitment to putting the public’s health first. The implementation of Republic Act 9502 was meant to provide affordable and accessible medicine to the public, and any attempts to obstruct this should be thoroughly investigated. It is essential that all parties involved act in a professional and ethical manner to ensure that the public’s health and well-being are not compromised.