Meropenem – AMSOPEN 1g
Meropenem – AMSOPEN 1g
Amsopen 1 g – Meropenem Powder for IV Injection/Infusion
-
Active ingredient: Meropenem trihydrate, equivalent to 1 g anhydrous meropenem per vial sahar.ph+11verification.fda.gov.ph+11myhealthbox.eu+11
-
Formulation: White to pale‑yellow sterile powder in a glass vial, with sodium carbonate (~90 mg) as a stabilizer
-
Packaging: Each box contains one vial plus two 10 mL sterile diluent ampoules.
Clinical Use & Administration
-
Antibacterial spectrum: Broad coverage against Gram-positive, Gram-negative, and anaerobes—ideal for empiric therapy in serious or resistant infections
-
Indications: Hospital/ventilator‑associated pneumonia, intra‑abdominal infections, complicated UTIs, bacterial meningitis, skin/soft tissue infections, febrile neutropenia, bacteremia in children ≥3 months and adults
-
Dosage: Typically 1 g IV every 8 hours in adults; pediatric and renal‑adjusted dosing also applicable (e.g., 10–40 mg/kg q8h in children, modified for kidney function).
Safety & Precautions
-
Common side effects: Diarrhea, nausea, headache, rash, elevated liver enzymes; risk of hypersensitivity reactions
-
Serious risks: Rare seizures (especially in renal impairment or CNS issues); monitor for allergic response unilab.com.ph
-
Storage & preparation: Store below 25 °C; reconstitute and infuse under sterile conditions; use promptly after reconstitution.
Amsopen 1 g – Meropenem Powder for IV Injection/Infusion
-
Active ingredient: Meropenem trihydrate, equivalent to 1 g anhydrous meropenem per vial sahar.ph+11verification.fda.gov.ph+11myhealthbox.eu+11
-
Formulation: White to pale‑yellow sterile powder in a glass vial, with sodium carbonate (~90 mg) as a stabilizer
-
Packaging: Each box contains one vial plus two 10 mL sterile diluent ampoules.
Clinical Use & Administration
-
Antibacterial spectrum: Broad coverage against Gram-positive, Gram-negative, and anaerobes—ideal for empiric therapy in serious or resistant infections
-
Indications: Hospital/ventilator‑associated pneumonia, intra‑abdominal infections, complicated UTIs, bacterial meningitis, skin/soft tissue infections, febrile neutropenia, bacteremia in children ≥3 months and adults
-
Dosage: Typically 1 g IV every 8 hours in adults; pediatric and renal‑adjusted dosing also applicable (e.g., 10–40 mg/kg q8h in children, modified for kidney function).
Safety & Precautions
-
Common side effects: Diarrhea, nausea, headache, rash, elevated liver enzymes; risk of hypersensitivity reactions
-
Serious risks: Rare seizures (especially in renal impairment or CNS issues); monitor for allergic response unilab.com.ph
-
Storage & preparation: Store below 25 °C; reconstitute and infuse under sterile conditions; use promptly after reconstitution.
New Arrival
Featured Products
Related products
-
Injectables
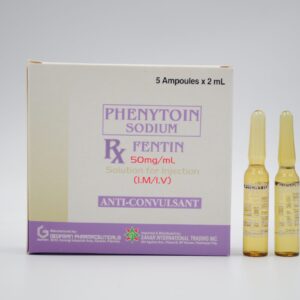
PHENYTOIN SODIUM (FENTIN) 50 mg/mL
-
Injectables
Piperacillin+Tazobactam (EFFEZOLIN) 4g/500mg
-
Injectables
TRAMADOL (SITIDOL) 50 mg/mL
-
Injectables
HYOSCINE BUTYLBROMIDE 20MG/ML (SPASMOCIN)
-
Injectables
HYOSCINE-N-BUTYLBROMIDE 20 MG/ML (ADUSCINE)