Multivitamins + Dextrose – Sitirose 250mL
Multivitamins + Dextrose – Sitirose 250mL
What it is:
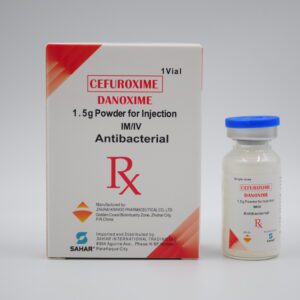
A prescription-only intravenous infusion combining carbohydrates (dextrose) with essential B‑vitamins and vitamin C.
Formulation per 250 mL: mercedesscientific.com+6verification.fda.gov.ph+6al-dawaa.com+6
-
25 g Dextrose: Primary calorie and energy source.
-
250 mg D‑Panthenol (Pro-vitamin B5)
-
125 mg Ascorbic Acid (Vitamin C)
-
25 mg Thiamine HCl (Vitamin B1)
-
25 mg Riboflavin (Vitamin B2)
-
25 mg Pyridoxine HCl (Vitamin B6)
-
625 mg Nicotinamide (Vitamin B3)
-
12.5 g additional Dextrose
Primary uses:
-
Supplementing nutritional and energy needs in patients unable to take oral intake
-
Correcting vitamin deficiencies (B‑complex & vitamin C) during IV therapy
-
Supporting recovery in cases of malnutrition, after surgery, or in critical illness
Manufacturer & approval:
-
Made by Shijiazhuang No. 4 Pharmaceutical Co., Ltd. (China)
-
Marketed and distributed in the Philippines by Sahar International Trading, Inc.
-
FDA‑PH registration valid from Dec 12, 2024 to Dec 12, 2029 verification.fda.gov.ph
What it is:
A prescription-only intravenous infusion combining carbohydrates (dextrose) with essential B‑vitamins and vitamin C.
Formulation per 250 mL: mercedesscientific.com+6verification.fda.gov.ph+6al-dawaa.com+6
-
25 g Dextrose: Primary calorie and energy source.
-
250 mg D‑Panthenol (Pro-vitamin B5)
-
125 mg Ascorbic Acid (Vitamin C)
-
25 mg Thiamine HCl (Vitamin B1)
-
25 mg Riboflavin (Vitamin B2)
-
25 mg Pyridoxine HCl (Vitamin B6)
-
625 mg Nicotinamide (Vitamin B3)
-
12.5 g additional Dextrose
Primary uses:
-
Supplementing nutritional and energy needs in patients unable to take oral intake
-
Correcting vitamin deficiencies (B‑complex & vitamin C) during IV therapy
-
Supporting recovery in cases of malnutrition, after surgery, or in critical illness
Manufacturer & approval:
-
Made by Shijiazhuang No. 4 Pharmaceutical Co., Ltd. (China)
-
Marketed and distributed in the Philippines by Sahar International Trading, Inc.
-
FDA‑PH registration valid from Dec 12, 2024 to Dec 12, 2029 verification.fda.gov.ph
New Arrival
Featured Products
Related products
-
Injectables
CEFTRIAXONE (GAVIXONE) 1 g
-
Injectables
METHYLERGOMETRINE MALEATE (ERGOMET)
-
Injectables
OMEPRAZOLE (NAHAZOLE) 40 mg
-
Injectables
OMEPRAZOLE (SIDPRAZOLE) 40 mg
-
Injectables
HYOSCINE BUTYLBROMIDE 20MG/ML (SPASMOCIN)