OMEPRAZOLE (NAHAZOLE) 40 mg
OMEPRAZOLE (NAHAZOLE) 40 mg
| GENERIC NAME: Omeprazole (as Sodium) |
| BRAND NAME: Nahazole |
| DOSAGE STRENGTH : 40 mg |
| DOSAGE FORM : Lyophilized Powder for Injection (IV) |
| PHARMACOLOGIC CATEGORY: Proton Pump Inhibitor |
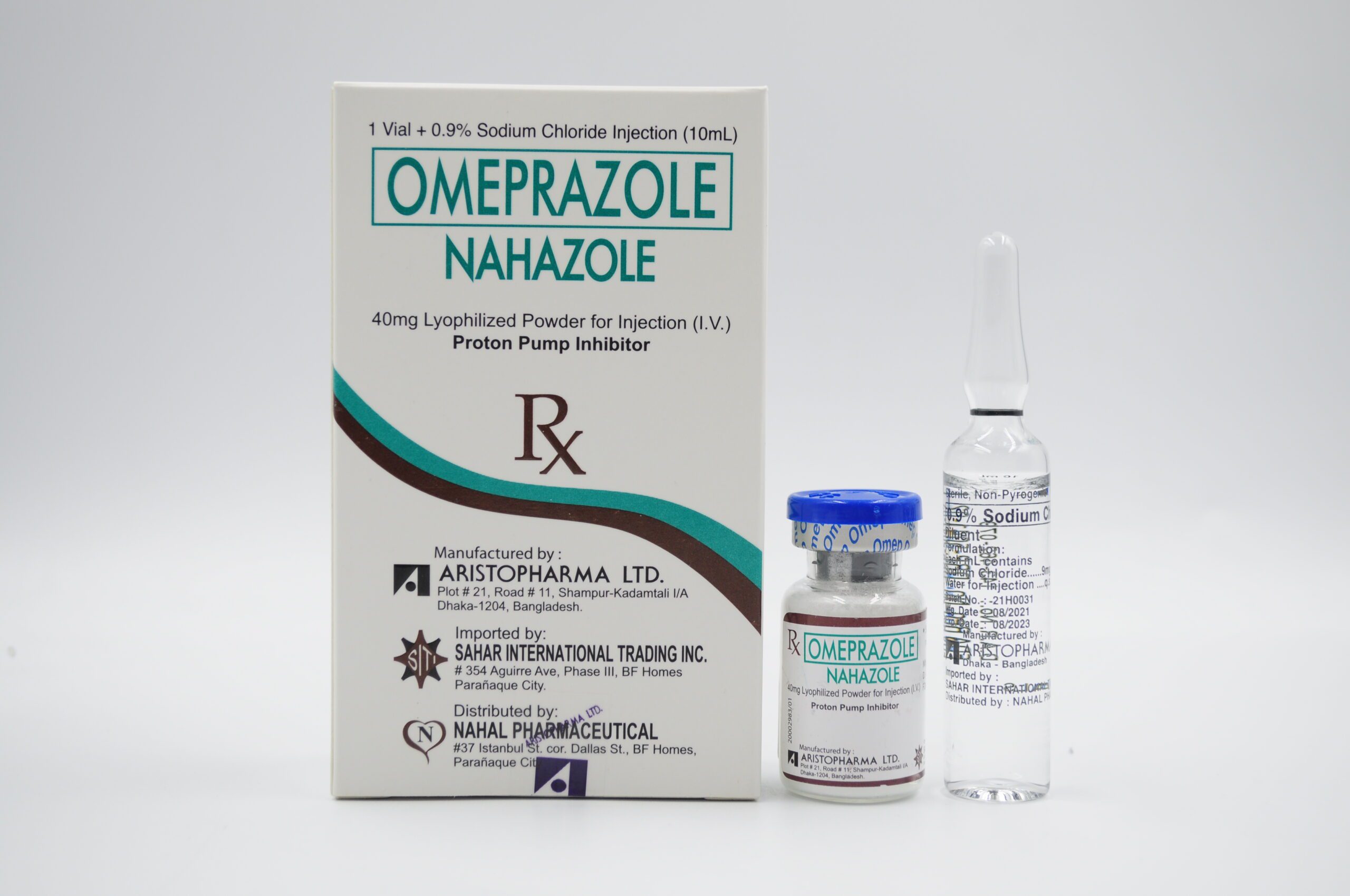
| INDICATIONS: Nahazole, a lyophilized powder for intravenous injection containing Omeprazole as Sodium at 40 mg strength. This formulation stands as a dependable treatment in managing acid-related conditions. Renowned for its precision and efficacy, Nahazole offers a swift and potent solution for issues like gastric ulcers, GERD, and other acid-related concerns. Crafted specifically for intravenous administration, this formulation ensures rapid relief and effective management of gastric acid hypersecretory disorders. Trusted by healthcare professionals, Sidprazole represents a vital tool in addressing acid-related ailments, promising relief and aiding in the overall management and comfort of patients. |
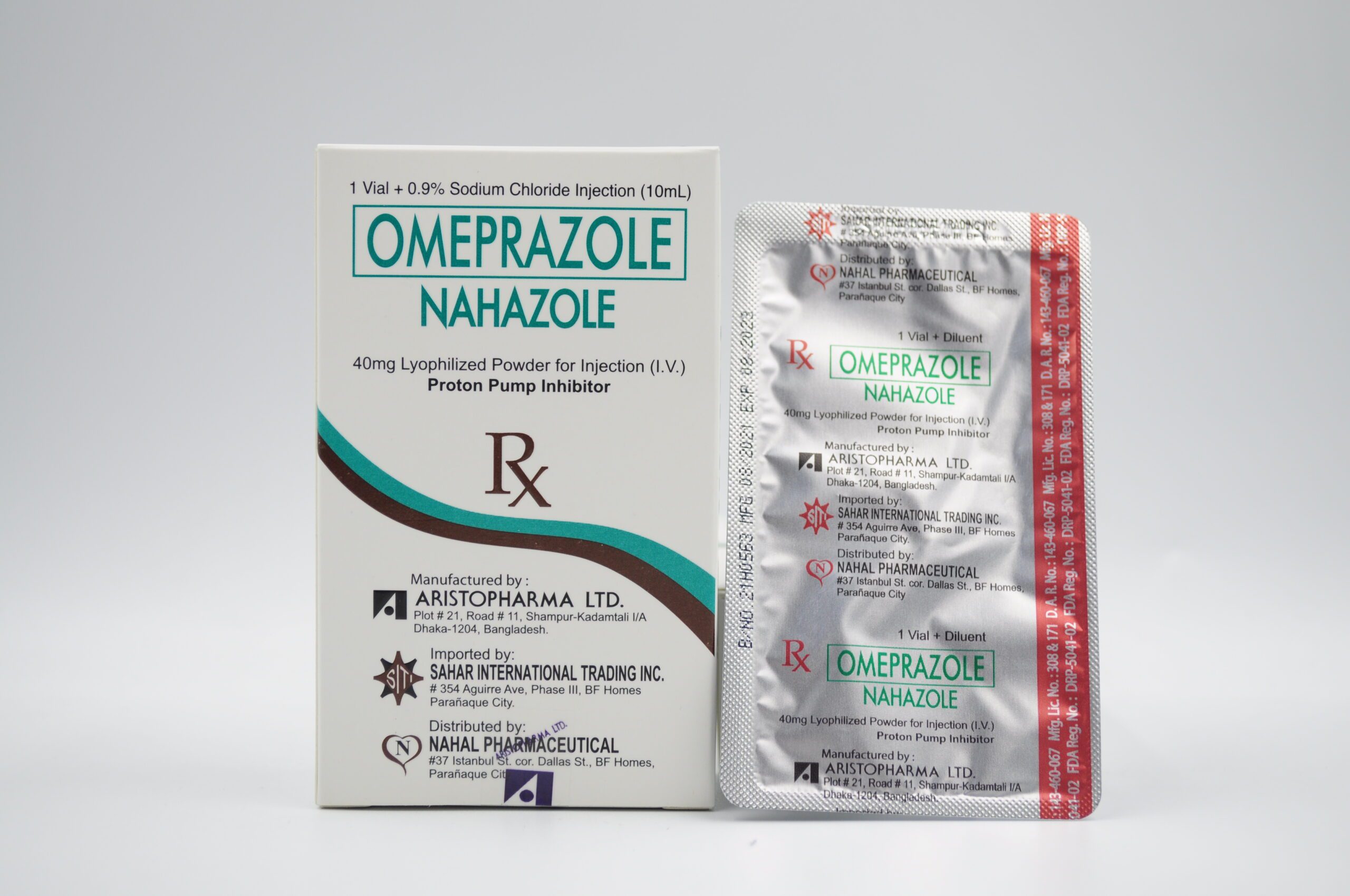
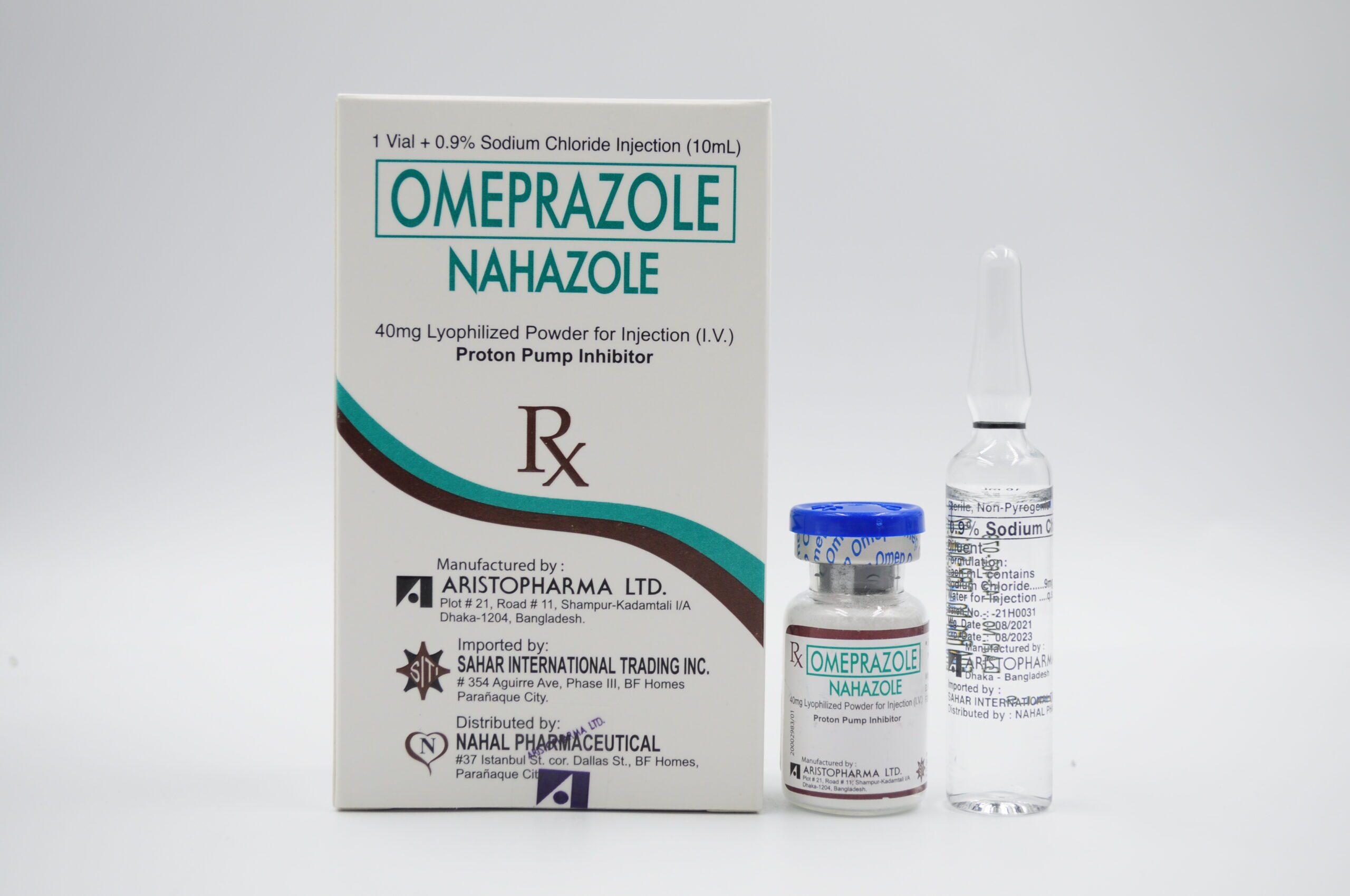
| PACKAGING: 10 mL USP Type I clear and colorless glass vial + 10 mL USP Type I clear and colorless glass ampoule (0.9% sodium chloride as diluent) (Box of 1s) |
| REG. NUMBER: DRP-5041-02 |
| GENERIC NAME: Omeprazole (as Sodium) |
| BRAND NAME: Nahazole |
| DOSAGE STRENGTH : 40 mg |
| DOSAGE FORM : Lyophilized Powder for Injection (IV) |
| PHARMACOLOGIC CATEGORY: Proton Pump Inhibitor |
| INDICATIONS: Nahazole, a lyophilized powder for intravenous injection containing Omeprazole as Sodium at 40 mg strength. This formulation stands as a dependable treatment in managing acid-related conditions. Renowned for its precision and efficacy, Nahazole offers a swift and potent solution for issues like gastric ulcers, GERD, and other acid-related concerns. Crafted specifically for intravenous administration, this formulation ensures rapid relief and effective management of gastric acid hypersecretory disorders. Trusted by healthcare professionals, Sidprazole represents a vital tool in addressing acid-related ailments, promising relief and aiding in the overall management and comfort of patients. |
| PACKAGING: 10 mL USP Type I clear and colorless glass vial + 10 mL USP Type I clear and colorless glass ampoule (0.9% sodium chloride as diluent) (Box of 1s) |
| REG. NUMBER: DRP-5041-02 |
New Arrival
Featured Products
Related products
-
Injectables
CEFEPIME HYDROCHLORIDE (MAXEF) 1 g
-
Injectables
CEFOXITIN (AMSOWEL) 1 g IM/IV
-
Injectables
Cefuroxime as Sodium (DANOXIME) 750 MG
-
Injectables
Tetanus Antitoxin (IMATET) 1500 IU/0.7 mL
-
Injectables
HYOSCINE BUTYLBROMIDE 20MG/ML (SPASMOCIN)